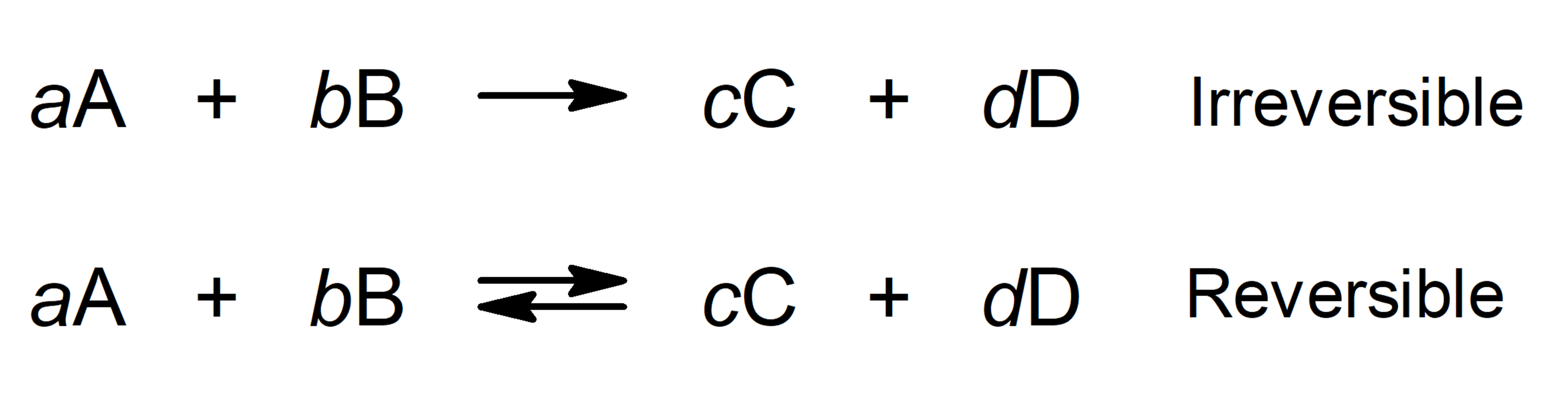Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution . in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. Describe the basic properties of solutions. why don't we take the effects of stirring and shaking on the rates of chemical reactions? stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). Describe how changing the temperature,. describe rate in terms of the conditions of successful collisions.
from www.exampleslab.com
in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. Describe how changing the temperature,. Describe the basic properties of solutions. describe rate in terms of the conditions of successful collisions. why don't we take the effects of stirring and shaking on the rates of chemical reactions? stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent.
20 Examples of Chemical Reactions Examples Lab
Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Describe the basic properties of solutions. why don't we take the effects of stirring and shaking on the rates of chemical reactions? stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. describe rate in terms of the conditions of successful collisions. stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. Describe how changing the temperature,. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions:
From www.teacharesources.com
Grade 8 Chemical reactions in animated PowerPoint. • Teacha! Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. why don't we take the effects of stirring and shaking on the rates of chemical reactions? stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. Describe how changing. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions & Solution Stoichiometry PowerPoint Presentation ID2145131 Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe the basic properties of solutions. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. Describe how changing the temperature,. stirring increases the rate of dissolution. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From dokumen.tips
(PDF) 04 types of chemical reactions & solution stoich DOKUMEN.TIPS Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. in the sections that follow, we discuss three of the most important kinds of reactions that occur. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From studylib.net
CHEM 1405 Chapter 7 Chemical Reactions.doc Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe the basic properties of solutions. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. why don't we take the effects of stirring and shaking on. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.chegg.com
Solved Part B Many chemical reactions in your body can Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe how changing the temperature,. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Describe the basic properties of solutions. in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. stirring and agitating chemical reactions is. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.slideserve.com
PPT CH. 3 Matter Properties and Changes PowerPoint Presentation, free download ID5741280 Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe how changing the temperature,. why don't we take the effects of stirring and shaking on the rates of chemical reactions? Describe the basic properties of solutions. describe rate in terms of the conditions of successful collisions. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a).. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free download ID6255486 Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.studocu.com
Chapter 6 Types of Chemical Reactions Solution Stoichiometry CHE 1301 Baylor University Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. describe rate in terms of the conditions of successful collisions. stirring and agitating chemical reactions is. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.slideserve.com
PPT STOICHIOMETRY PowerPoint Presentation, free download ID3997530 Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. describe rate in terms of the conditions of successful collisions. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). in the sections that follow, we discuss three. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.studypool.com
SOLUTION Chemy 101 chapter 4 reactions in aqueous solutions Studypool Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). describe rate in terms of the conditions of successful collisions. stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. in a reflux setup, solvent vapors are trapped. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From studylib.net
typesofchemicalreactions Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: describe rate in terms of the conditions of successful collisions. Describe how changing the temperature,. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). why. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From chemistnotes.com
Oscillating chemical reactions Easy Definition, 3 examples Chemistry Notes Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From sites.google.com
01. Types of Reactions IST Science Department Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe how changing the temperature,. describe rate in terms of the conditions of successful collisions. in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: stirring. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From thewonderofscience.com
Chemical Reactions — The Wonder of Science Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe how changing the temperature,. stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. describe rate in terms of the conditions of successful collisions. why don't we. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From studylib.net
AP* Chemistry TYPES OF CHEMICAL REACTIONS & SOLUTION Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution why don't we take the effects of stirring and shaking on the rates of chemical reactions? in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. in a. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.dreamstime.com
Young Scientist Doing Chemical Reactions in the Laboratory Stock Image Image of dioxide Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution Describe how changing the temperature,. describe rate in terms of the conditions of successful collisions. Describe the basic properties of solutions. stirring affects how quickly a solute dissolves in a solvent, but has no effect on how much solute will dissolve. stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From www.storyboardthat.com
Identifying Signs of Chemical Reactions StoryboardThat Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution stirring increases the rate of dissolution because it helps to distribute the solute particles evenly throughout the solvent. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). Describe how changing the temperature,. describe rate in terms of the conditions of successful collisions. in the sections. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.
From chemistrytalk.org
Chemical Reactions Infographic ChemTalk Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution describe rate in terms of the conditions of successful collisions. stirring and agitating chemical reactions is desirable and stirring reflux systems or any system under heating is necessary to a). in a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the. Describe the basic properties of solutions. Describe. Why Is It Important To Stir During The Process Of Many Chemical Reactions In Solution.